605-646-3325
info@cellfield.tech
The Quest for Non-Animal Pre-Clinical Models: Navigating Biotech's Greatest Challenge
The Pioneering Spirit of Biotech
In the realm of biotechnology, the quest for non-animal pre-clinical models represents a frontier rife with both promise and challenge. Unlike treatments that merely alleviate symptoms, non-animal pre-clinical models aim to halt or reverse disease progression, offering hope for definitive solutions to chronic conditions. Osteoarthritis (OA), a prevalent and debilitating joint disease, serves as a compelling case study in this ambitious pursuit. Despite the growing need, the path to developing these models for OA and similar diseases is fraught with scientific, regulatory, and economic obstacles.
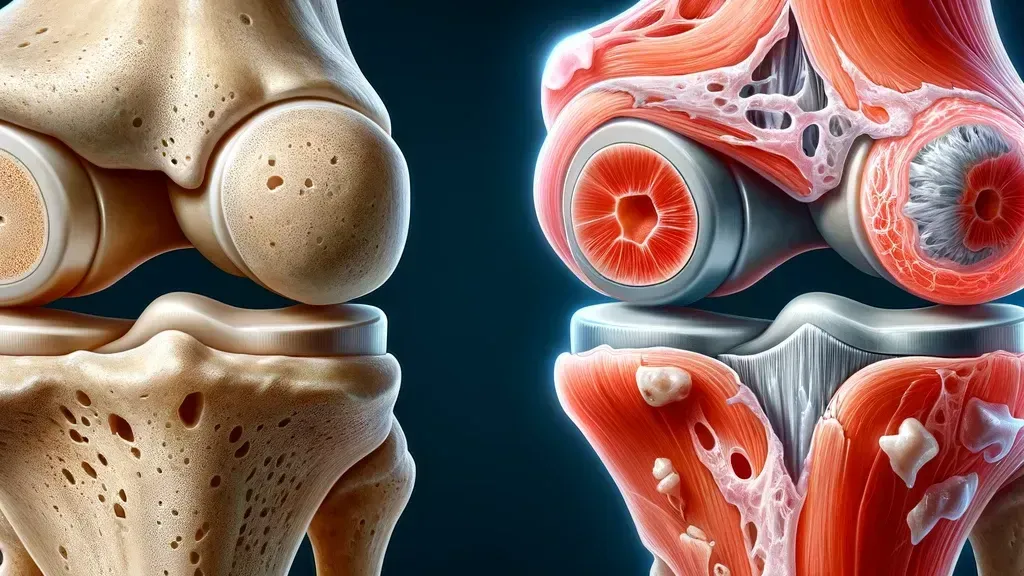
Understanding Osteoarthritis: OA is characterized by the breakdown of cartilage, leading to pain, stiffness, and loss of mobility. It affects millions worldwide, yet no FDA-approved non-animal pre-clinical models exist to counteract its progression. This gap underscores a critical challenge: designing therapies that can effectively target the complex biological mechanisms underlying OA.
The Biotech Hurdle: Complexity and Cost
Scientific Complexity: The intricate pathology of OA, involving cartilage degradation, bone remodeling, and inflammation, complicates the identification of therapeutic targets. Preclinical Testing advancements, while promising, often struggle to replicate the multifaceted nature of human joint degeneration.
Economic Barriers: Developing non-animal pre-clinical models is a costly endeavor, with research and development expenses running into billions. For conditions like OA, the long duration of drug trials and the need for extensive patient monitoring amplify these costs.
Regulatory Pathways: Navigating the regulatory landscape presents another layer of complexity. Demonstrating a drug's disease-modifying capabilities requires robust, long-term clinical evidence, a task that poses significant logistical and financial challenges.
Despite these hurdles, biotech firms are forging ahead with innovative approaches to development. Biotechnology Advancements in genomics and proteomics have begun to unravel the molecular intricacies of OA, offering new targets for intervention. Micro-physiological systems and In Vitro Models are revolutionizing preclinical testing, enabling more nuanced exploration of drug effects on human tissue.
Highlighting success stories provides invaluable insights into overcoming development challenges. For instance, recent breakthroughs in gene therapy and stem cell treatments offer a glimpse into potential OA interventions. These examples not only demonstrate scientific feasibility but also shed light on strategic partnerships and funding models that have facilitated progress.
A Call to Action for the Biotech Community
The journey toward non-animal pre-clinical models for osteoarthritis and beyond is emblematic of the broader challenges facing the biotech industry. Collaboration between academic researchers, biotech companies, and regulatory bodies will be pivotal in navigating the complexities of development. Moreover, embracing innovative research methodologies and technologies promises to accelerate the discovery and deployment of these much-needed therapies.
The quest for non-animal pre-clinical models is more than a scientific endeavor; it's a call to action for the entire biotech community. By fostering collaboration, investing in innovation, and navigating regulatory challenges with agility, we can turn the tide against debilitating diseases like osteoarthritis. The path is arduous, but the potential rewards—a future where chronic diseases can be halted or reversed—are immeasurably valuable.
Biotech News